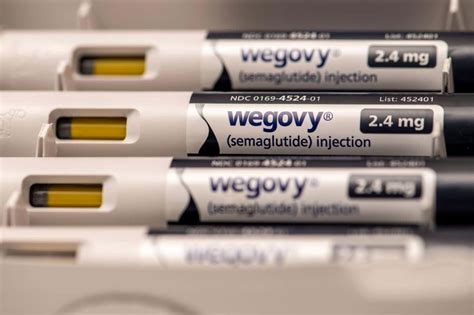
In a surprising turn of events, the Trump administration recently made a significant decision regarding healthcare coverage. It was revealed that the administration rejected a proposal put forth by the Biden presidency to expand Medicare’s coverage to include obesity drugs like Wegovy and others. This move has sparked debates and discussions across the country about healthcare policies and access to essential medications.
**The Rejection Decision**
The rejection came as a reversal of the previous administration’s plan that aimed to provide millions of Americans with access to weight-loss drugs through Medicare and Medicaid. This decision has stirred mixed reactions from different quarters, with supporters and opponents voicing their opinions on the matter.
Biden’s Proposal
Under President Biden’s proposed expansion, popular weight-loss drugs would have been covered by Medicare and Medicaid. This move was estimated to cost billions of dollars due to the sheer number of individuals who would benefit from this extended coverage. The plan faced challenges due to existing regulations that prohibited Medicare from funding drugs specifically for “weight loss.”
Cost Implications
Expanding coverage for obesity drugs under government healthcare programs such as Medicare would have incurred substantial costs. The Congressional Budget Office projected an expense of approximately $35 billion over a decade if the proposal had been implemented.
Regulatory Updates
The recent decision not only concerned the coverage of obesity drugs but also formed part of a comprehensive 438-page regulation update impacting various aspects of Medicare’s Part D drug benefits and Medicare Advantage plans. These changes are crucial in shaping the future landscape of healthcare services provided under these programs.
**Expert Insights**
Experts in healthcare policy have weighed in on this development, highlighting potential implications on public health outcomes and financial considerations. Driven by differing perspectives, professionals within the industry continue to analyze how this decision will impact patients requiring obesity treatment options.
**Future Policy Considerations**
While current indications suggest a denial of expanded coverage for obesity drugs, there remains room for future policy revisions concerning these medications. The Centers for Medicare and Medicaid Services expressed openness towards reevaluating their stance on this matter, leaving the door open for potential shifts in policy direction down the line.
**Conclusion**
As discussions surrounding healthcare accessibility persist, decisions like these underscore the complex nature of balancing medical needs with financial constraints within government-funded programs like Medicare and Medicaid. The evolving landscape of healthcare regulations continues to shape how individuals access vital treatments while navigating intricate policy frameworks set forth by successive administrations.







Leave feedback about this